|
|
Hot
Topics: Going vertical: Gauging ocean overturn rate
We on Expedition 7 talk a lot about finding deep-sea
corals, but we’re especially interested in them for what they
can tell us about climate change over time. To get that information,
we need to sample corals from a long vertical column of different
ocean depths.
This means that while gathering a lot of samples is important, it’s even
more important that those samples represent many different depths in the ocean,
from relatively shallow to relatively deep. This is because water in the ocean
has different ages at different depths.
The age of a water mass is defined by the time since
it last “saw” the surface. Because the starting point
for deep ocean circulation is the North Atlantic, water is “age
zero” there and gets “older” as it moves closer
to the North Pacific.

The global ocean
circulation system transports heat worldwide. Learn how differences
in the temperature and saltiness of seawater drives deep ocean
circulation. Learn more » As it takes that journey, the water carries with
it carbon that is undergoing radioactive decay. This decay can
help us determine the age of the water it lies in, and from that
we can measure how long water takes to circulate through the entire
ocean and then come back to the surface. This is called the ocean’s “overturn
rate”.
So how does this radioactive decay work? Carbon has three forms, or isotopes:
12C, 13C, and 14C, where the different numbers refer to different atomic weights.
14C is unstable, meaning that it undergoes radioactive decay; 12C is stable,
which makes it a handy reference point when the 14C decays. They have the same
chemistry, but only 14C is lost through time.
We measure the rate of decay with a term called a half-life. 14C’s half
life is 5,730 years. This means that every 5,730 years, there’s half as
much 14C as there was in the previous 5,730-year period. To extend this concept,
in 11,460 years, there’s one-fourth the amount of 14C as there was originally.
At this point you might be asking how decaying carbon got into the ocean in the
first place. The answer to that question is at the heart of what makes Expedition
7 so compelling. All radiocarbon is produced in the upper atmosphere by cosmic
rays. So, all radiocarbon in the ocean must have come from the sky and entered
at the surface. This occurs through the exchange of carbon dioxide (CO2) gas
between the surface ocean and the atmosphere.
The radiocarbon is then distributed throughout the ocean’s depths, but
unequally. At the surface of the ocean there is the same 14C/12C ratio as in
the atmosphere. In the deep part of the ocean, where the water and its carbon
haven’t seen the top for a while, there’s a lower ratio of 14C
to 12C, or more radioactive decay.
The difference between radiocarbon levels in the deep and shallow sections of
the ocean is a measurement of the time it takes for the ocean to turn over. The
longer the ocean takes to overturn, the larger the difference between radiocarbon
concentration at the top and the bottom.
A measurement in the modern ocean of the vertical distribution of radiocarbon
lets you know how long since each layer was at the top. Today, that number is
about 1,000 years for the average ocean. At the end of the circulation path in
the North Pacific, there is 25 percent less radiocarbon than in the atmosphere.
Deep-sea corals, in a way no other climate archive can, record the
14C make-up of the ocean in which they lived, because they make their
skeletons of calcium carbonate. This makes them ideal tools for us
to measure the ocean’s rate
of overturn in the past.
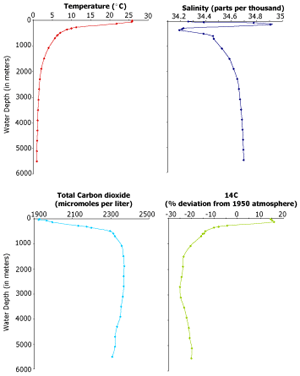 These
graphs show vertical profiles through the water column in the Northwest These
graphs show vertical profiles through the water column in the Northwest
Pacific. The top two show temperature and salinity. The bottom two show
total dissolved carbon dioxide and a measure of the deviation in 14C from
a 1950 atmosphere (that is a comparison that scientists make). The difference
in 14C in the water at different layers is related to how long ago that
water was at the surface of the ocean. The lower the value, the older the
water.
Back to Main Hot Topics Page
|

