|
Hot Topics: Dating Corals, Knowing the Ocean Locating corals in the deep ocean and retrieving them presents all sorts of challenges. But once they’re on board, what good are they? For Expedition 7, much of the corals’ value lies in what they can tell us about the past -- recalling that history presents its own set of challenges.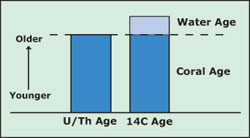 The uranium-thorium “age” of a coral is the age of the coral. The radiocarbon (14C) “age” of the coral is the age of the coral, plus the age of the water in which it grew. Subtracting the former from the latter gives the age of the water in which it grew. Scientists use this information to learn about the rates at which water circulates through the oceans. Carbon is a useful element for dating objects because it’s so prevalent in our environment. The unstable form, or isotope, of carbon is 14C; its stable, unchanging isotope is 12C, where the different numbers refer to different atomic weights. As 14C decays, the ratio of 14C to 12C changes over time. This change allows us to measure age. We measure the rate of radioactive decay with what’s called a half-life.
14C’s half life is 5,730 years. This means that every 5,730 years, there’s
half as much 14C as there was in the previous 5,730-year period. To extend
this concept, in 11,460 years, there’s one-fourth the amount of 14C
as there was originally. The global ocean circulation system transports heat worldwide. Learn how differences in the temperature and saltiness of seawater drives deep ocean circulation » Ordinarily, we measure the age of an object by comparing the current 14C/12C
ratio with ratio of the past atmosphere, since that is where radioactive carbon
comes from. The difference between the two is the age since it was formed.
But with deep-sea corals, that difference is both the age since the coral was
formed and the age of the water in which it grew.
|
|
© 2010 Dive and Discover™. Dive and Discover™ is a registered trademark of Woods Hole Oceanographic Institution
|
|
