Dating Corals, Knowing the Ocean
Locating corals in the deep ocean and retrieving them presents all sorts of challenges. But once they’re on board, what good are they? For Expedition 7, much of the corals’ value lies in what they can tell us about the past -- recalling that history presents its own set of challenges.
The uranium-thorium “age” of a coral is the age of the coral. The radiocarbon (14C) “age” of the coral is the age of the coral, plus the age of the water in which it grew. Subtracting the former from the latter gives the age of the water in which it grew. Scientists use this information to learn about the rates at which water circulates through the oceans. In order to know anything about past climate from corals, we need to know their age. Ordinarily, scientists determine an object’s age by measuring theradioactive decay of some element in it. Radioactive decay occurs when an unstableform of that element changes into a stable one by spinning off a part of itsnucleus.
Carbon is a useful element for dating objects because it’s so prevalent in our environment. The unstable form, or isotope, of carbon is 14C; its stable, unchanging isotope is 12C, where the different numbers refer to different atomic weights. As 14C decays, the ratio of 14C to 12C changes over time. This change allows us to measure age.
We measure the rate of radioactive decay with what’s called a half-life. 14C’s half life is 5,730 years. This means that every 5,730 years, there’s half as much 14C as there was in the previous 5,730-year period. To extend this concept, in 11,460 years, there’s one-fourth the amount of 14C as there was originally.
Ordinarily, we measure the age of an object by comparing the current 14C/12C ratio with ratio of the past atmosphere, since that is where radioactive carbon comes from. The difference between the two is the age since it was formed. But with deep-sea corals, that difference is both the age since the coral was formed and the age of the water in which it grew.
Since we want to know both of these values, we face the classic problem of having one measurement and two unknowns. In such cases, you need to somehow determine one of those unknowns from another angle. In the case of the deep-sea corals, we get their age by analyzing another element they contain: uranium.
Like carbon, uranium is radioactive. As it decays, it changes into another element, thorium. Fortunately, while a coral is growing it incorporates a lot of uranium but no thorium. This means that as it ages its thorium/uranium ratio increases at a known rate. So, measurements of the thorium/uranium ratio are measurements of the coral’s age.
Now we know two things: time since the coral was formed (from uranium), and the sum of that time and the past water mass’s age (from carbon). So, the difference between these two gives us the past radiocarbon age of the water.
Collecting fossil corals at different depths is like collecting water profiles today. The coral records in its skeleton all that we need to know. We just have to find the ways to tease it apart.
Locating corals in the deep ocean and retrieving them presents all sorts of challenges. But once they’re on board, what good are they? For Expedition 7, much of the corals’ value lies in what they can tell us about the past -- recalling that history presents its own set of challenges.
The uranium-thorium “age” of a coral is the age of the coral. The radiocarbon (14C) “age” of the coral is the age of the coral, plus the age of the water in which it grew. Subtracting the former from the latter gives the age of the water in which it grew. Scientists use this information to learn about the rates at which water circulates through the oceans. In order to know anything about past climate from corals, we need to know their age. Ordinarily, scientists determine an object’s age by measuring theradioactive decay of some element in it. Radioactive decay occurs when an unstableform of that element changes into a stable one by spinning off a part of itsnucleus.
Carbon is a useful element for dating objects because it’s so prevalent in our environment. The unstable form, or isotope, of carbon is 14C; its stable, unchanging isotope is 12C, where the different numbers refer to different atomic weights. As 14C decays, the ratio of 14C to 12C changes over time. This change allows us to measure age.
We measure the rate of radioactive decay with what’s called a half-life. 14C’s half life is 5,730 years. This means that every 5,730 years, there’s half as much 14C as there was in the previous 5,730-year period. To extend this concept, in 11,460 years, there’s one-fourth the amount of 14C as there was originally.
Ordinarily, we measure the age of an object by comparing the current 14C/12C ratio with ratio of the past atmosphere, since that is where radioactive carbon comes from. The difference between the two is the age since it was formed. But with deep-sea corals, that difference is both the age since the coral was formed and the age of the water in which it grew.
Since we want to know both of these values, we face the classic problem of having one measurement and two unknowns. In such cases, you need to somehow determine one of those unknowns from another angle. In the case of the deep-sea corals, we get their age by analyzing another element they contain: uranium.
Like carbon, uranium is radioactive. As it decays, it changes into another element, thorium. Fortunately, while a coral is growing it incorporates a lot of uranium but no thorium. This means that as it ages its thorium/uranium ratio increases at a known rate. So, measurements of the thorium/uranium ratio are measurements of the coral’s age.
Now we know two things: time since the coral was formed (from uranium), and the sum of that time and the past water mass’s age (from carbon). So, the difference between these two gives us the past radiocarbon age of the water.
Collecting fossil corals at different depths is like collecting water profiles today. The coral records in its skeleton all that we need to know. We just have to find the ways to tease it apart.
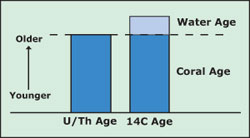
The uranium-thorium “age” of a coral is the age of the coral. The radiocarbon (14C) “age” of the coral is the age of the coral, plus the age of the water in which it grew. Subtracting the former from the latter gives the age of the water in which it grew.




